Medibio Clinical Study Shows Over 20% Improvement to Current Diagnostic Standard
September 6, 2018 | By Medibio Team
Highlights:
– Global 230 patient (8 sites) study confirms Medibio’s Depression Diagnostic Aide (DX04)
– With 70% accuracy, 70% sensitivity and 71% specificity results demonstrate utility in long-term monitoring
– Study results support the FDA DeNovo submission
– Diagnostic algorithm designed to provide mental health practitioners with an objective technology to aide in assessment of depression
– Demonstrated positive predictive value up to 72% and negative predictive value up to 78%
Sydney, Australia and Minneapolis, MN USA – 6 September 2018: Medibio Limited (MEB or the Company)(ASX: MEB)(OTCQB: MDBIF), a mental health technology company, today announced results from the pivotal clinical study evaluating the accuracy of its clinical prediction algorithm. Results show statistical accuracy of the algorithm to detect a Major Depressive Episode (MDE). This validation is a significant achievement in the company’s development of an objective test for aiding in the diagnosis of depression in patient care. The Company is excited by the findings of the clinical study and believes the results and medical needs for our technology support our FDA DeNovo application.
Key benefits of the clinical study results include:
1. Multiple intended scientific publications and support of the FDA DeNovo submission
2. Shows effectiveness as an adjunctive diagnostic aide
3. Accuracy of 70% with 70% sensitivity and 71% specificity
4. Results indicate 20-40% improvement from current diagnostic standard [1,2,3,4]
5. Patent-pending algorithm provides sophisticated and accurate measurements; potential for long-term monitoring of mental disorders
6. Study targeted general population in a normal-daily-home environment
Clinical Study’s Medical Benefit
The technology is a step forward in the search for objective digital biomarkers of depression and other mental illnesses supported by clinical prediction models, taking into consideration that inter-rater disagreement is common in mental health conditions. Among the most common reasons for discord are: interpretation variances (39%), followed by information variance (30%), criterion variance (27%), and observation variance (4%). [5]
Medical benefits of the Medibio algorithm is the improvement of diagnostic sensitivity and specificity, and reduction of under-diagnosis, as compared to patients who only received clinical assessments. The results provide further validation of Medibio’s proposition that psychiatric conditions differentially affect the autonomic nervous system (ANS), resulting in anomalies of cardiac and sleep functions. This proposition is based on over 15 years of research investigating ANS disturbances linked to mental state and their observation via the cardiovascular system during sleep when external influences are absent.
Clinical Study Results
The Depression Diagnostic Aide (DX04) study was a global, pivotal, case-controlled trial designed to evaluate and accurately classify a patient as having a depressive episode or not. The study was conducted in eight sites across the United States and Australia. During the study, subjects were clinically diagnosed for neuropsychiatric disorders and assessed by the algorithm. The primary efficacy endpoint was based on specificity and sensitivity. As a secondary efficacy endpoint, the repeatability of the algorithm was used to establish accuracy in identifying the presence of MDE.
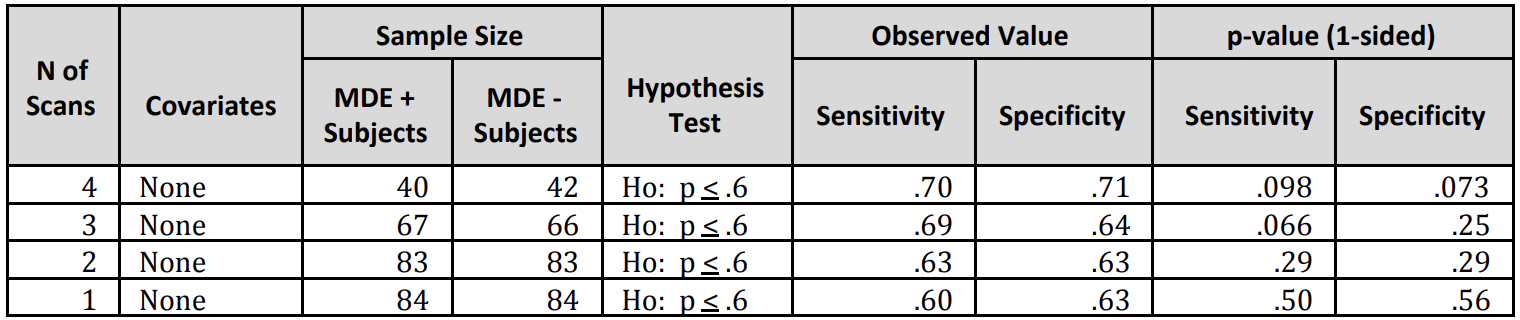
For patients that completed a full-course monitoring of four scans (over two weeks), the sensitivity and specificity to detect and monitor MDE symptoms was 70%-71% – AUC 0.7 vs. single and shorter recordings 60%-63% – AUC 0.6. When covariates such as age and sex were added, the sensitivity and specificity were 70%-69% – AUC 0.8 vs 65%-62% – AUC 0.7, respectively. Using the first of four continuous scans and a prevalence sample size balance of 60%, the positive predictive value is 72% and the negative predictive value is 78%, both with a 95% confidence interval.
Medibio’s algorithm distinguished accurately between individuals with MDE and non-depressed controls in 70%-71%. The algorithm is designed to provide mental health practitioners with an objective technology to aide in assessment of MDE. These results are superior to known depressive inter-rater agreement values that range from 0.64-0.48 [1,2,3,4].
Along with the clinical study protocol and results, the FDA review of the Company’s full application also includes medical claims, intended use, instructions for use, safety, efficacy, usability, and labeling claims. The technology is part of the Medibio platform which aides diagnosis of other mental health disorders.
The following table represents results of the clinical study:
Early development phases of this algorithm, including the previously reported MACH-3 pilot study results (August 2017), were used to support this hypothesis with respect to initial clinical study protocol and functionality. Prior studies were based on a low hypothesis rate, had low study subjects, used a limited number of study sites, and were completed in controlled environments.
Comparison to Prior Studies
This current multi-center clinical trial (DX04) is the pivotal study to support FDA filing for the technology. The study enrolled 230 patients at 8 clinical study sites and was designed to evaluate the technology’s performance for detecting MDE compared to the subjective interview of a physician. In the study, the algorithm was able to correctly identify the presence of MDE 70% of the time, and was able to correctly identify those patients who did not have MDE 71% of the time.
About Medibio Limited
Medibio (ASX: MEB) (OTCQB: MDBIF) is a mental health technology company that has pioneered the use of objective digital biomarkers to assist in the screening, diagnosing, monitoring and management of depression and other mental health conditions. The company was founded in Australia, with offices located in Melbourne (Vic), Perth (Wa) and U.S. offices in Minneapolis, MN. Medibio is listed on the Australian Securities Exchange Ltd and trades on the OTCQB Venture Market. Investors can find additional information on www.otcmarkets.com and www.asx.com.au
| Further Information: | |
| Medibio Enquiries:
Josh Purdy Senior Public Relations Manager Medibio Limited T: +1 952 314 1216 |
Australian Media Enquiries:
Peter Taylor NWR Communications peter@nwrcommunications.com.au T: +61 (0) 412 036 231 |
Sources:
1. Mulsant BH., et al. Interrater reliability in clinical trials of depressive disorders. AM J Psychiatry. 2002 Sept: 159(9): 1598-1600.
2. Lieblich Samuel, et.al. High heterogeneity and low reliability in the diagnosis of major depression will impair the development of new drugs: BJPsych Open (2015) 1, e5–e7.
3. Cairney J., et al. Evaluation of 2 measures of psychological distress as screeners for depression in the general population. The Canadian Journal of Psychiatry 2007 Feb: Vol 52(2).
4. Einfeld S., et al. Inter-Rater Reliability of the Diagnoses of Psychosis and Depression in Individuals with Intellectual Disabilities: 2007 Sept: 20 (5): 384-390
5. Kobak KA,. et al. Sources of unreliability in depression ratings. J Clin Psychopharmacol. 2009 Feb: 29(1) 82-85.



